How Surfactants Work and Why You Should Use Them
Spray adjuvants and surfactants can significantly increase the effectiveness of your pesticide applications. Here is how they work and why greenkeepers should consider adding spray adjuvants and surfactants to their turf management toolkit.
What is a spray adjuvant or surfactant?
An adjuvant is a substance added to a spray tank to modify the chemical or physical properties or characteristics of a product in application. The addition of certain adjuvant products in applications of herbicides, insecticides and fungicides has the potential to directly or indirectly increase pesticide performance or to reduce risk associated with application.
Spray adjuvants can be divided into two categories; activators and spray modifiers, and utility modifiers. Activators and spray modifiers work to directly improve the biological performance of the pesticide, whereas utility modifiers do not directly improve pesticide performance, but minimise the chance of loss of performance due to other variables. Each of these categories can be further broken down into the groups that we know in the market.
| Activators and Spray Modifiers | Utility Modifiers |
| Surfactants | Buffering agents |
| Crop Oils | Drift control agents |
| Stickers | Foam reduction agents |
| Spreaders | Compatibility agents |

Why should you use a spray adjuvant?
There are countless variables in the application of pesticides, including environmental conditions, applicator experience, water quality and the application method. All of which have the potential to impact pesticide performance, or to pose risk to humans or the environment. Some publications state that up to 70% of pesticide performance depends on the success of the spray application. With so many variables surrounding the act of pesticide application, it is vital to ensure that all bases are covered if the desired result is to be achieved.
Losses before application
- Chemical reactions in the spray tank - usually caused by hard or dirty water can begin to reduce the quality of the pesticide immediately after tank mixing. Some cations (particularly Ca, Mg, Na, and Fe) attach to the negatively charged pesticide molecules forming a complex. This essentially locks up the pesticide molecule, so that it is unable to complete its intended purpose. Dirty water containing suspended particles can also cause some pesticide molecules (e.g. glyphosate) to lock up, again becoming useless.
- Alkaline hydrolysis – this can cause irreversible destruction of the pesticide in a solution with high pH (i.e. >7). Generally this occurs when the water used during application is of a high pH, which breaks down some susceptible pesticides over a period of time. Using acidifying agents to lower the pH of the water to a desirable range for the particular pesticide will prevent or minimise this occurrence
Losses during application
- Physical drift - caused by fine droplets and hot, dry or unstable conditions. As well as resulting in less on-target pesticide contact (and so reduced performance), this can also result in off-target damage of other organisms or environments. Drift control agents often reduce this by increasing the droplet size of spray or coating.
- Secondary loss from leaf surface - caused by bounce, shatter and runoff often occurs during application. Large droplets can often run off the target surface before the pesticide can act, also small droplets with high velocity have the potential to bounce off the target. Surfactants can reduce these effects by reducing the surface tension of the water allowing it to spread onto the target rather than bounce, shatter or run off.
Losses after application
- Metabolism by plant or insect targets – this occurs once the product enters the target and begins its activity. Systemic pesticides will begin to be transferred through the target organism and as they work, pesticide molecules will begin to degrade. By increasing the concentration of pesticide that is absorbed into the target, the product will remain for longer after the metabolism begins.
- Volatile loss from surfaces – some chemicals are volatile and following application can change from a liquid or a solid to a gaseous form. As a gas or vapour the pesticide can then move into the atmosphere, travelling great distances on the wind potentially resulting in off-target damage.
- Breakdown by sunlight, bacteria and other natural means – following application the pesticide molecules will be at the mercy of the environment, with rain, sunlight and some bacteria all acting to break the products down. Increasing the period of time that pesticide molecules can remain uncompromised by these environmental factors can reduce the potential for losses, and increase pesticide efficacy.
How do adujvants work?
Spray adjuvants increase the coverage of spray droplets by reducing surface tension
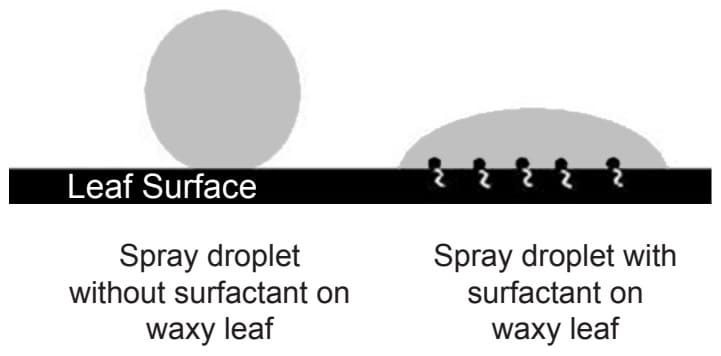
- Surfactants (surface active agents) work by reducing the surface tension of water in the pesticide solution. The result of this is an increase in the spreadability of the water, increasing the surface area that the solution can cover and the efficiency that it does so. This is why straight water on an impermeable surface (such as the waxy cuticle of a plant leaf ) will form a bead, rather than spread outwards. With the addition of a surfactant, water molecules are able to spread outwards as they are rearranged. This allows for increased efficiency in the solutions’ contact with the target, whether it is turf leaf area, weed plant or an insect pest.
- Straight water has a high surface tension as water molecules are strongly attracted to one another. These cohesive forces mean that water molecules are more inwardly attracted to themselves than they are outwardly attracted to molecules in surrounding materials, forming a spherical shape as their energy is concentrated inward. Surfactants have the ability to rearrange the water molecules and tip the balance for the molecules to be less attracted to themselves, and more so to external materials; in essence making water wetter. This occurs because surfactant molecules are both hydrophilic (water-loving) and lipophilic (oil-loving). The surfactant molecules link their lipophilic tails to the waxy surface of the leaf, and their hydrophilic heads to water molecules. With the addition of a surfactant, the water can now interact with other surfaces with the surfactant molecules acting as a link between the two otherwise non-compatible materials.
Spray adjuvants can increase the penetration of spray droplets
- Some adjuvants act to enhance the uptake of systemic pesticides or foliar-applied nutrients by improving cuticle penetration. Following application, the product polymerises and dries into an elastic film that encapsulates the pesticide particles. Systemic chemicals then move from the film into the plant tissue. As the adjuvant dries rapidly into the waterproof film, it protects systemically translocated chemicals until they can penetrate and enter plant tissue.
Spray adjuvants can increase the adherence or sticking of spray droplets
- Deposition (sticking) agents are adjuvants added to solution to increase the initial amount of pesticide deposited during field application and also to protect the spray residue from loss due to irrigation, rain, dew, leaf abrasion and wind erosion. Deposition agents will emulsify in a water solution and polymerize upon application to ensure spray materials do not migrate from target plant or soil surfaces. Contact insecticides and fungicides are slowly released on the outside of the film for re-distribution and active pest & disease control as the film naturally weathers.

Spray adjuvants can help reduce the degradation of spray solutions
- Some adjuvants work by altering the chemical quality of the spray water to minimise loss due to hard water or alkaline hydrolysis. Ammonium sulphate is often used to prevent hard water from impacting on pesticide performance. The sulphate ions (24%) in the product bind to the cations in the hard water preventing them from interacting with the pesticide. Acidifying agents will lower the pH of the solution to reduce alkaline hydrolysis. Reduction in degradation or breakdown of spray droplets
- Some adjuvants act as a sunscreen, preventing degradation of the pesticide by ultra violet light. Specialised products can encapsulate pesticides before they can evaporate, reducing volatilization and UV degradation. This allows the pesticide to be released slowly for more effective activity
Spray adjuvants reduce the foaming of spray solutions
- Many pesticides react when agitated and foam up making tank mixing and spray application difficult and sometimes inaccurate. Foam reduction agents work to disrupt the structure of the foaming liquids, returning them to a more stable solution. These can either be added beforehand to prevent foaming occurring, or afterwards to break down existing foam. These products generally have a very low viscosity and are insoluble. This allows them to spread out over the foam surface, where they then interact with the foam, destabilising the membrane and causing the collapse of the foam structure. This action then allows the solution to be applied as a standard liquid, unhindered by foam.











