Ninemire Irrigation Suitability
About the irrigation water analysis
The quality of water used to irrigate a turf surface will often reflect the health of the soil and the turf. Irrigation water varies significantly in their quality and all have unique characteristics. Over time, many of the characteristics will have a direct effect on certain aspects of soil nutrition, soil structure and plant health; these effects could be either positive or negative.
Nuturf's Irrigation Suitability Analysis will help you understand the unique characteristics of your irrigation source, to have the ability to fix problems in the water, before they begin affecting the soil, in turn reducing turf quality.
More About Nuturf's Irrigation Suitability Analysis
Issues associated with the condition of irrigation water often occur slowly over a long period of time and they can be difficult to remediate quickly, and may require substantial alterations to turf amendment programs and general management practices.
This is why it is important to know the unique characteristics of your irrigation source, to have the ability to fix problems in the water, before they begin affecting the soil, in turn reducing turf quality. The most important variables of a turf grass irrigation source are listed below with a brief description on the effects they can have on turf and soil quality.

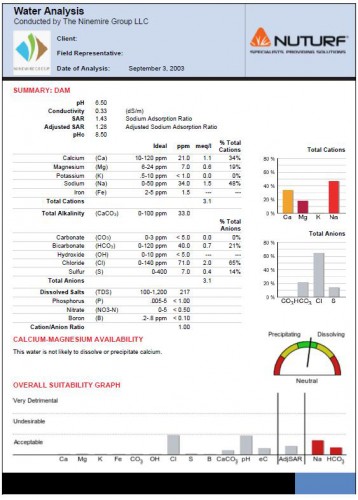
pH
pH is the measure of the acidity or alkalinity of the irrigation water. It is important to regularly monitor the pH level of an irrigation source; because low, high or fluctuating pH is often a symptom of other water quality issues. When it is established that there is a pH problem in the irrigation water, a full Irrigation Suitability Analysis is recommended to identify the cause.
The ideal pH for irrigation water is 6.5, levels much higher (alkaline) or lower (acidic) can alter the soil pH over time. This causes nutrients to become unavailable to the plant and in extremes can directly damage the turf foliage and roots.

Electrical Conductivity (EC) and Total Dissolved Salts (TDS)
The electrical conductivity (EC) of water is its ability to conduct a measured electric current between two probes. The higher the conductivity the more dissolved nutrients present. This measurement helps to give a level of the Total Dissolved Salts in the solution and therefore how nutrient rich the water is. It must be noted that EC is not a reading of sodium alone, but of all dissolved ions including beneficial nutrients such as nitrogen, phosphorous, potassium, calcium, magnesium, etc.
If water has a low TDS, the likelihood of it stripping nutrients from the soil profile following irrigation is high. When water is high in dissolved salts it is reluctant to pick up more nutrients as it percolates through the profile. This is because water behaves in a way where it will absorb elements to bring itself to a similar “density” as the matter around it.
pHc and Calcium and Magnesium Availability
pHc is a calculation which indicates whether or not the solution is likely to precipitate or dissolve cations (often Ca and Mg). A pHc below 8.5 means that the water is more likely to precipitate these cations; whereas a pHc above 8.5 means that the water is likely to dissolve them.
When cations are precipitated from the solution they become unavailable to the plant and are unable to offset sodium build-up or influence soil structure. When cations are dissolved, they are easily leached from the profile, again rendering them useless.
Total Cations – Calcium, Magnesium, Potassium, Iron, Sodium
Cations are the positively charged elements in the solution. The percentage of total cations should comprise of at least 50% calcium. Calcium is beneficial to plant health, and is the key to good soil structure. Calcium causes soil particles to be drawn together to create aggregates (flocculation), this in turn creates pore spaces and allows for air and water movement within the profile.
If an irrigation source regularly contains high sodium levels (above 120ppm) the reverse of the calcium effect can occur. Sodium in a soil profile causes soil particles to repel each other, creating a compact profile with little porosity.

Total Anions – Carbonate, Bicarbonate, Hydroxide, Chloride, Sulphur
Anions are the negatively charged elements in the solution; they generally have a negative effect on the soil and the plant when in high levels. Bicarbonates react with calcium and magnesium forming insoluble calcium carbonate (CaCO3) and magnesium carbonate (MgCO3), which can precipitate from the solution resulting in a loss of beneficial Ca and Mg.
Another anion that can be more directly damaging to turf health is chloride (Cl). In high levels chloride can be toxic to turf foliage and roots, where damage occurs at levels of about 250ppm. It can reduce photosynthesis and cause leaf burn, as well as reducing water uptake in the soil. Below are the hazard levels for anions.

Sodium Absorption Ratio (SAR) and Adjusted Sodium Absorption Ratio (Adj.SAR)
SAR is the measure of sodium (Na) in the solution in relation to Ca and Mg. Adj. SAR is the same test; however this measurement also takes into consideration the levels of carbonates and bicarbonates in the solution. By doing this the Adj.SAR test can estimate the likelihood of a reduction in available Ca and Mg that would limit the cations effectiveness in sodium displacement (due to bicarbonate precipitation or dissolving of cations). These tests can provide a hazard rating of how likely sodium build-up will be in the soil if irrigated with high sodium water. It is important to remember that if the waters bicarbonates are low, SAR should be used to determine sodium hazards. And if bicarbonates are high, then Adj.SAR must be used to ensure an accurate result.


